Skip to content
Home 12.1 Electrons in atoms
12.1 Electrons in atoms
Emission Spectra and ionization
- First ionization energy IE1 relates to process:
- Second IE is
- Nth ionization energy IEn relates to process:
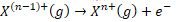
- IE1 < IE2 < IE3 < IE4 …
- In emission spectrum, the lines converge at higher energies. At the limit of convergence, the lines merge forming a continuum
- Beyond continuum, electron can have any energy
- The frequency of the radiation in the emission spectrum at the limit of convergence can be used to determine IE1
Periodic Trends in ionization energies
- In Ca, big jump between IE2 and IE3 because corresponds to removing electron from fully occupied 3p sublevel
- In Ti, big jump between IE4 and IE5 because change in energy level. Ti5+ does not occur naturally
- Ionization energy for Ti increases more gradually than Ca because electrons are being removed from 3d and 4s orbitals, which are much closer in energy than 3p and 4d.
- These big jumps in energy occur when removal of electrons from different energy levels n (1,2,3,4,…)
