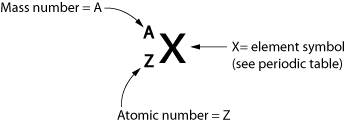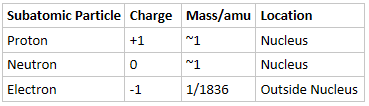Home 2.1 The nuclear atom
2.1 The nuclear atom
Atom
- Atoms consist of a three subatomic particles
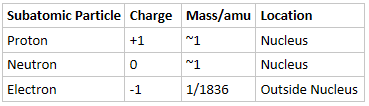
- Atomic Number Z: number of protons in the nucleus of an atom of an element.
- Mass Number A: number of protons + number of neutrons
- Relative atomic mass Ar: Ratio of the average mass of the atom to the unified atomic mass unit
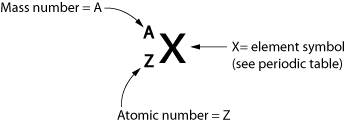
- Atomic Mass Units (AMU): 1/12th of the mass of a carbon – 12 atom in its ground state. This is used to express masses of atomic particles.
- 1 AMU = 1.6605402 x 10-27 kg
The mass spectrometer
- The mass spectrometer is an instrument used to determine the relative atomic mass Ar of an element by using its isotopes.
- Watch this video