Types of isomerism
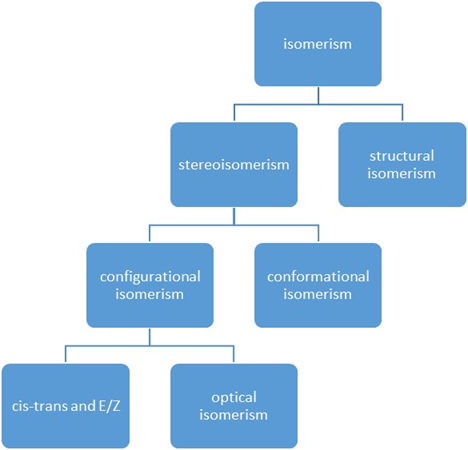 Isomers: compounds composed of the same elements in the same proportions but differ in properties because of differences in the arrangement of atoms
Isomers: compounds composed of the same elements in the same proportions but differ in properties because of differences in the arrangement of atoms
Structural Isomers: share same molecular formula but atoms bonded in completely different ways
Stereoisomers: have an identical molecular formula and bond multiplicity but show different spatial arrangements of the atoms
Conformational isomers: can be interconverted by rotation about the σ bond
Configurational isomer: can only be interconverted by breaking and reforming the σ or π bond
Cis-trans and E/Z:
- Cis-trans is a a sub category of E/Z isomers. Cis-trans is used when the groups in concern are exactly the same
- Cis-trans and E/Z are found in alkenes and cycloalkanes only
- In the case below, the group in concern is CH3
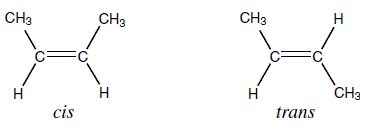
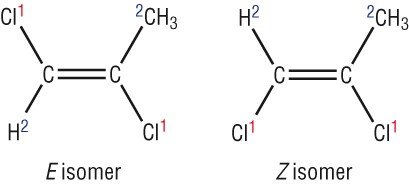
- To determine E/Z isomers, first observe left side of the double bond. The group with the highest atomic number has the most priority. Then repeat the same step on the right side. Finally, determine whether it is E or Z configuration depending on which sides the groups with the most priority are on.
- Cis is equivalent to Z, trans is equivalent to E
Optical isomers:
- Determined by the presence of chiral carbon also known as stereocentres
- Chiral carbon is a carbon bonded to 4 different atoms or groups of atoms
- The * represents a chiral carbon
- Optical isomers have ability to rotate and exist in pairs called enantiomers or diastereomers
- Enantiomers: Mirror images of each other. Have 1 or more chiral carbons.
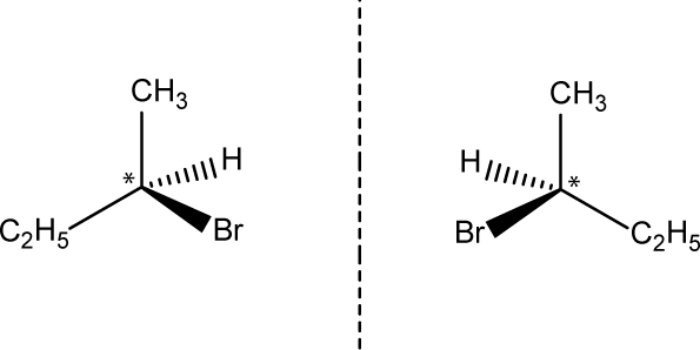
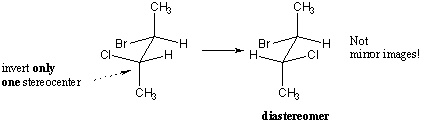
- Diastereomers: Not mirror images of each other. Have 2 or more chiral carbons.
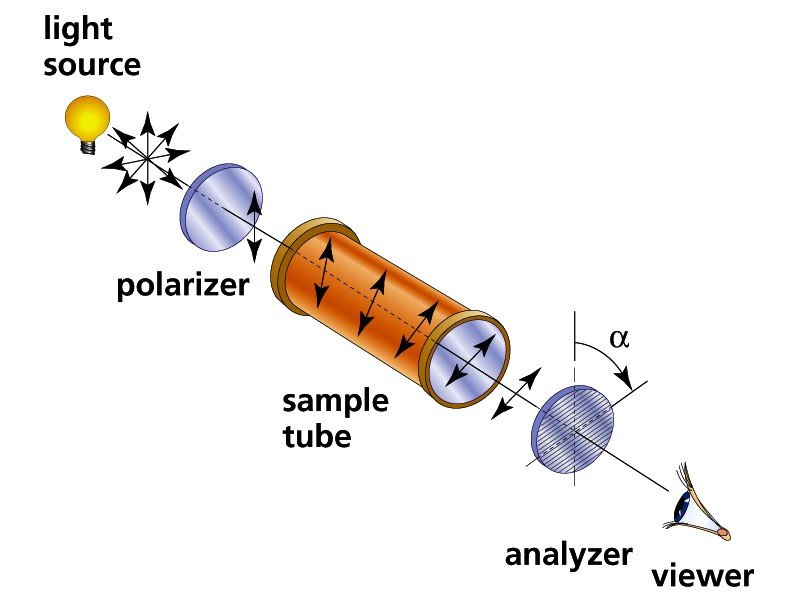
Optical isomers and plane polarize light
- The optical activity of enantiomers can be detected and measured by an instrument called a polarimeter
- Consists of light source, two polarizing lenses, and a tube between the lenses to hold sample of enantiomer dissolved in a suitable solvent
- Light first passes through polarizer and becomes plane-polarized (vibrating in a single plane)
- Rotating the analyzer (clockwise is dextrorotary, anticlockwise is laevorotary) lets more light in through the analyzer
- If both enantiomers are present in equal amounts, the two rotations cancel each other out and is optically inactive. Such a mixture is called a racemic mixture or racemate