Home 6.1 Collision theory and rates of reaction
6.1 Collision theory and rates of reaction
- Chemical Kinetics: The study and discussion of chemical reactions with respect to reaction rates
- Rate of Reaction: The change in concentration of reactants or products per unit time.
Experimental measurements of reaction rates
- Δc, the change in concentration can be measured indeirectly by monitoring a property which changes when reactants are converted to products. Examples include:
- pH (acid – base reactions)
- Conductivity (reactions with electrolytes)
- Mass/volume (reactions involving gases)
- Colour (reactions with transition metals or other colored compounds)
- To measure reaction rate, plot concentration vs time graph. The rate is determined from slope of gradient at point t on the graph.
- Rate of reaction can be measured in three ways
- Average rate
- Instantaneous rate
- Slope of tangent at a point
- Initial rate
- Slope of tangent at point t0
Kinetic molecular theory of gases
- Explains why gases act the way they do
- Theory
- Gases have many particles moving at high velocities in random directions
- Size of a gaseous particle is negligible
- Collisions between gaseous particles are elastic; no energy is lost
- Keav is proportional to the absolute temperature in kelvin
Occam’s razor
- Used as a guide to develop a theory
- A principle which states that “Entities should not be multiplies unnecessarily”
- This means if you have two competing theories, use the simpler one unless there is proof otherwise
- Collision theory was built using Occam’s razor
Catalysts
- A substance that increases the rate of a chemical reaction by lowering the activation energy and is not consumed in the reaction.
- This can be demonstrated using a potential energy profile
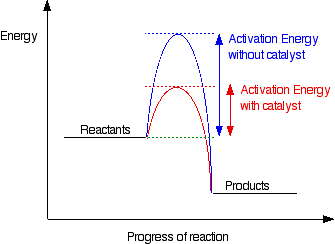
- Activation energy: The minimum energy required for a reaction to occur
- Catalysts come in two types:
- Homogeneous:
- In the same physical state as the reactants
- Example is destruction of ozone, O3, by chlorine atoms. In the stratosphere, ozone absorbs over 95% of UV radiation from sun. It under goes homolytic fission and converts UV radiation to heat. Chlorine atoms are produced in the reaction of a chlorofluorocarbon (CFC) with UV light.
- Heterogeneous:
- In a different state from the reactants
- Example is catalytic converter in exhaust system of car which converts harmful gaseous into water oxygen or carbon dioxide
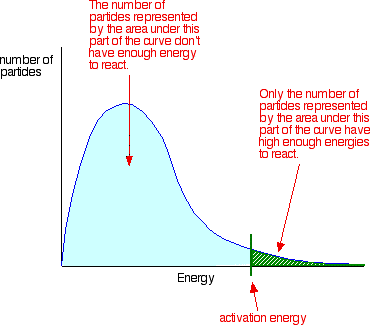
Maxwell – Boltzmann energy distribution and temperature
- Shows the probability of finding particles with a specific kinetic energy
- Number of particles represented by area of green (in the picture) have enough energy to react
- For a catalyzed reaction, the activation energy decrease, and there are more particles which have enough energy to react
- As temperature increases, the mean velocity of particles increases and thus the distribution become flatter and wider
Factors that affect the rate of chemical reaction
- Increasing temperature
- Gives particles more kinetic energy, faster rate of reaction
- Addition of catalyst
- Reduces activation energy, faster rate of reaction
- Increasing concentration of reactants
- Increasing concentration means more collisions, faster rate of reaction
- Increasing surface area in solid phase
- Breaking down solid into smaller pieces means larger overall SA, more collisions, faster rate of reaction


