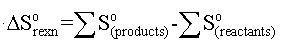Entropy: A measure of the distribution of available energy among the particles. The more ways energy can be distributed, the higher the entropy. (The greater the shift from energy being localized to being widespread amongst the particles, the lower the chance of the particles returning to their original state and the higher the entropy of the system)
Spontaneous changes
- Spontaneous reaction: A reaction which moves towards completion or equilibrium under a set of given conditions without external intervention
- Enthalpy is one aspect considered in measuring spontaneity of a reaction
- Entropy increases as state changes from solid to liquid to gas
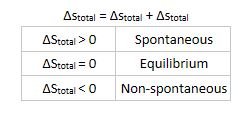
- Second law of thermodynamics says that chemical reactions that result in increase of entropy of universe are spontaneous.
Calculating Entropy changes
Gibbs free energy
- An equation to combine enthalpy, entropy, and temperature of a system
- ΔGѳ = ΔHѳ – TΔS
- For reaction to be spontaneous, Gibbs Free energy must have negative value
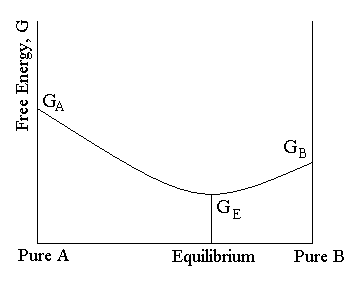
Gibbs free energy and chemical equilibrium
Calculations
- Gibbs free energy change of formation:

- Represents the free energy change when 1 mol of a compound is formed from its elements under standard conditions
- If the Gibbs free energies of formation are not known, use enthalpy and entropy data.
- ΔGѳ = ΔHѳ – TΔS
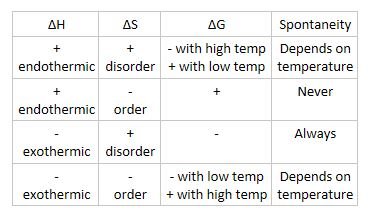
Affect of positive and negative values on Gibbs free energy