Home 13.1 First-row d-block elements
13.1 First-row d-block elements
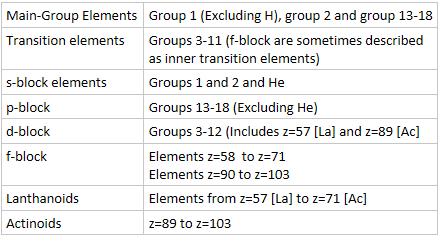
- Transition elements are sometimes described as transition metals due to their metallic nature.
- Important: Two exceptions are Chromium and Copper. Cr electron configuration is [Ar]3d54s1 and not [Ar]3d44s2. Cu electron configuration is [Ar]3d104s1 and not [Ar]3d94s2.
Characteristics of Transition Elements:
- Variable oxidation states
- Can have oxidation states ranging from +1 to +7
- This is due to patterns of successive ionization energies
- Compounds of transition elements and their ions are often coloured
- g. KMnO4 is purple
- g. Cu2SO4 . H2O is blue
- Compounds of zinc are colourless unless ligands have chromophore (group of atoms responsible for absorption of electromagnetic radiation)
- Transition metals form complexes with ligands
- Coordinate bonds between metal is bonded to group of atoms/molecules (termed ligands)
- See classification of ligands below
- Transition metals are often used as catalysts
- g. (Haber process, biological catalysts)
- Magnetic properties of transition metals depend on their oxidation states and coordination number
- Depend on things such as oxidation state, coordination number, and the geometry of complex.
- Paramagnetic contain unpaired electrons that behave as tiny magnetics
- Diamagnetic do not contain unpaired electrons and therefore are repelled by external magnetic fields
Classification of Ligands
- Ligands are either negatively charged anions Cl– or neutral molecules with lone pair (e.g. NH3)
- Number of coordinate bonds depends on number of lone pairs in ligand which are bonded
- Monodentate ligands are 1 coordinate bond actually bonded. Examples include H2O and NH3
- Polydentate (chelate) ligands can form 2 or more. Examples include (EDTA)4- which can form 6 bonds
Coordination Numbers
- Total number of points of attachment to central element.

