Home 9.2 Electrochemical cells
9.2 Electrochemical cells
Electrochemical Cells
- In an electrochemical cell, there can either be a conversion of chemical energy to electrical energy, or the other way around
- There are two main types of electrochemical cells
- Voltaic (Galvanic) Cells – convert chemical to electrical energy. Reactions are spontaneous and exothermic.
- Electrolytic Cells – convert electrical to chemical energy. Non spontaneous.
Electrodes
- An electrode is a conductor of electricity used to make contact with a non-metallic part of a circuit, such as the solution in a cell (electrolyte)
- Electrochemical cells contain two electrodes, anode and the cathode
- In both voltaic and electrolytic cells:
- Oxidation is at the anode
- Reduction is at the cathode
- Mnemonic is “An Ox, red cat“
- In voltaic cell
- Cathode is positive
- Anode is negative
- In electrolytic cell
- Cathode is negative
- Anode is positive
Cell Diagram
- Cell Diagrams are used as shorthand notation to represent a voltaic cell. By convention, anode is always of left, and cathode on right. The salt bridge is represented by two parallel lines
- Zn(s)|Zn2+(aq)||Cu2+(aq)|Cu(s)
Types of Cells
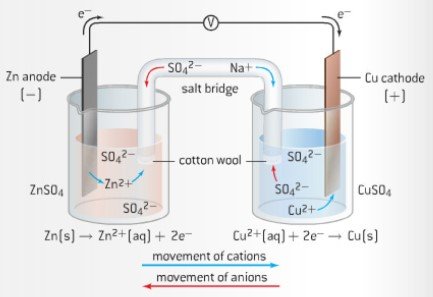
- Voltaic (Galvanic) Cells
- Consists of two half-cells. These two are separated, connected only by a salt bride. Oxidation occurs at one half-cell (anode), reduction occurs at on half-cell (cathode)
- There are different types of electrodes used in voltaic cells, most common one is metal/metal ion electrode
- Metal/metal ion electrode
- Consists of a bar of metal placed in a solution containing cations of the same metal. Examples include:
- Zn(s)|Zn2+(aq)
- Cu(s)|Cu2+(aq)
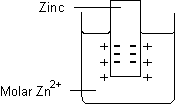
- The vertical line represents a phase boundary (junction)
- Salt bridge which connects both electrodes has multiple functions:
- Provides physical separation of reduction and oxidation processes
- Provides electrical continuity for anions and cations
- Reduces the liquid-junction potential. This is the voltage generated when two different solutions come into contact with each other
- Salt bridge contains a concentrated solution of a strong electrolyte. The high concentration allows ions to diffuse out of it. The ions in a salt bridge must be inert
- To determine which metal will be oxidized, or which will be reduced, refer to the activity series. Zinc is higher up on series than copper thus it is more easily oxidized. The zinc half-cell acts as the anode.
- Voltaic Cell example:
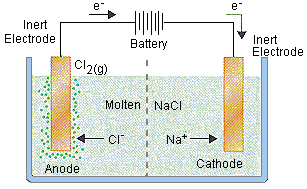
- Electrolytic Cells
- Electrolysis is the process by which electrical energy is used to drive a non-spontaneous chemical reaction
- Electrolytic cells consist of a container of electrolyte, two electrodes, and a battery which is considered an electron pump
- There are many types of electrolytic cells, most common one is molten salt cell
- Molten Salt Electrolysis
- Identify all species
- Identify species attracted to cathode (negative electrode) and anode (positive electrode)
- Deduce the two half-reactions taking place at each electrode, and overall cell reaction
- Draw and annotate electrolytic cell and show direction of electrons and of ions
- State what would be observed at each electrode
- Electrolytic Cell example: