Home D.2 Aspirin and penicillin
D.2 Aspirin and penicillin
Aspirin
- The first active ingredient, salicylic acid, was isolated from the bark of willow tree and used for pain relief. However, pure salicylic acid caused sever digestive problems such as bleeding, diarrhea. These side effects were reduce by chemical modification to create acetylsalicylic acid or aspirin
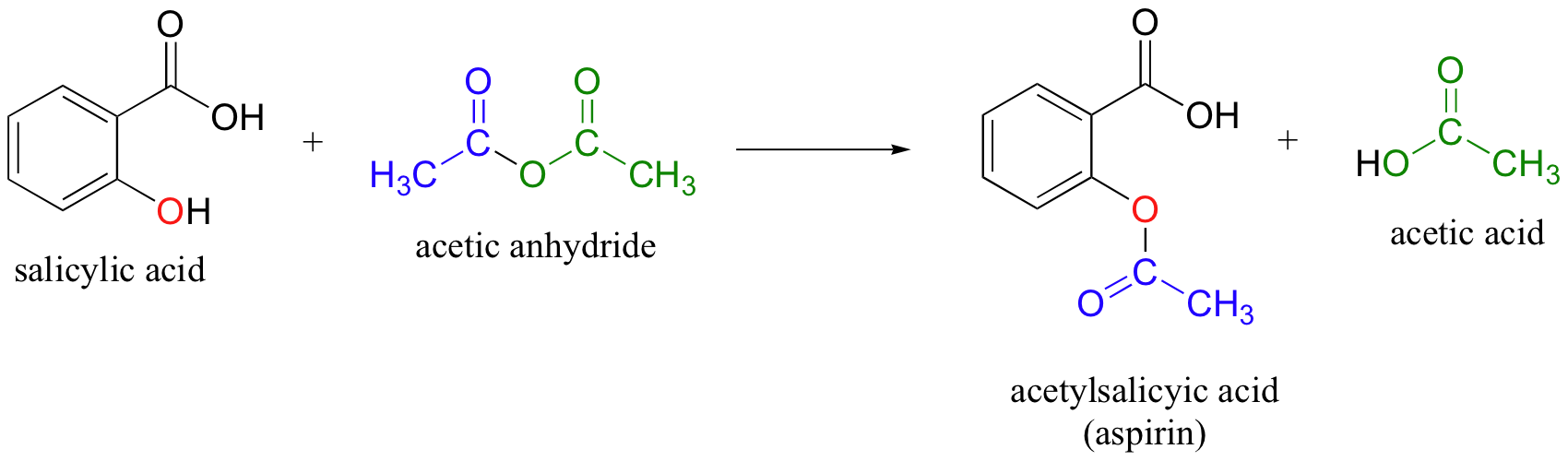
- In a typical experiment, salicylic acid is mixed with excess ethanoic anhydride and several drops of catalyst (concentrate phosphoric acid)
- The mixture is then heated, diluted with water, cooled to produce crystals. The crystals are then recrystallized in hot ethanol
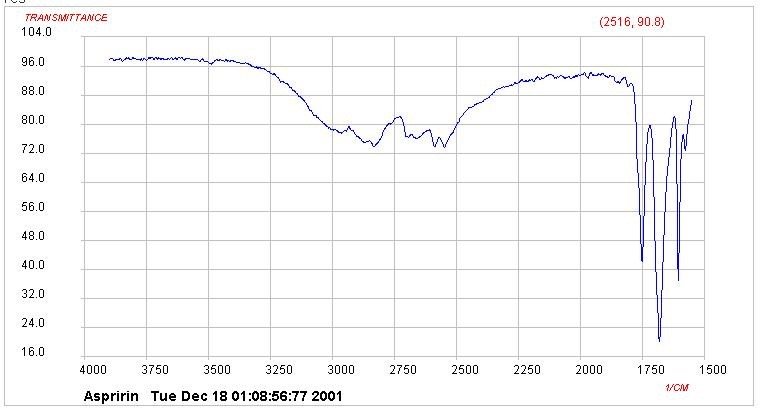
- Aspirin can be identified by IR spectroscopy and melting point (136°C)
- Below is general shape for identity of aspirin
Effects of Aspirin
- Aspirin and salicylic acid belong to a class of mild analgesics, also known as non-narcotic analgesics and non-steroidal anti-inflammatory drugs (NSAIDs)
- Two other mild analgesics are paracetamol and ibuprofen. These are prefer in countries due to reduced side effects
- Mild analgesic: A drug which acts to reduce mild pain
- Mild analgesics also reduce fevers and inflammation caused by irritation
- In contrast to strong analgesics, mild analgesics affect nervous system by intercepting pain stimulus at its source. Aspirin particular binds to enzyme cyclooxygenase and suppresses production of prostaglandins, which are responsible for fever, and transmission of pain impulses from site of injury to brain
- Prostaglandins also produce thromboxanes, which ultimately results in platelets causing clots. Thus aspirin acts as an anticoagulant, reducing risk of strokes and heart attacks caused by clotting. However anticlotting leads to excessive bleeding
- Risk of stomach bleeding increases when aspirin is taken together with alcohol (ethanol). This synergistic effect is example of drug interaction
- Other side effects of aspirin include allergies, acidosis (decrease in pH of blood) and Reye’s syndrome in young children (fatal liver and brain damage)
Solubility of Aspirin
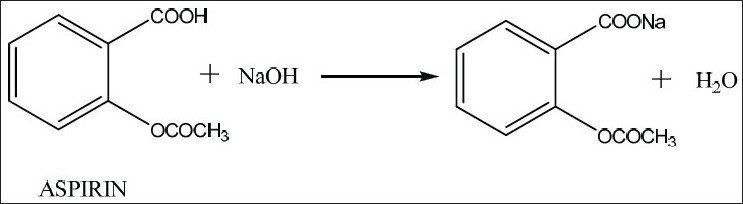
- Since aspirin is almost insoluble, its bioavailability is limited
- The solubility and thus bioavailability of drugs can be increase by converting them into ionic salts
- In aspirin, the carboxylic group can be neutralized with sodium hydroxide, producing soluble sodium salt of aspirin
Penicillin
- A bacteriologist mistakenly left a petri dish open and noticed a blue-green mold developed which prevented the growth of his bacteria. This antibacterial fungus later became known as penicillin
- This antibiotic drug saved more lives across the world than any other pharmaceutical drug
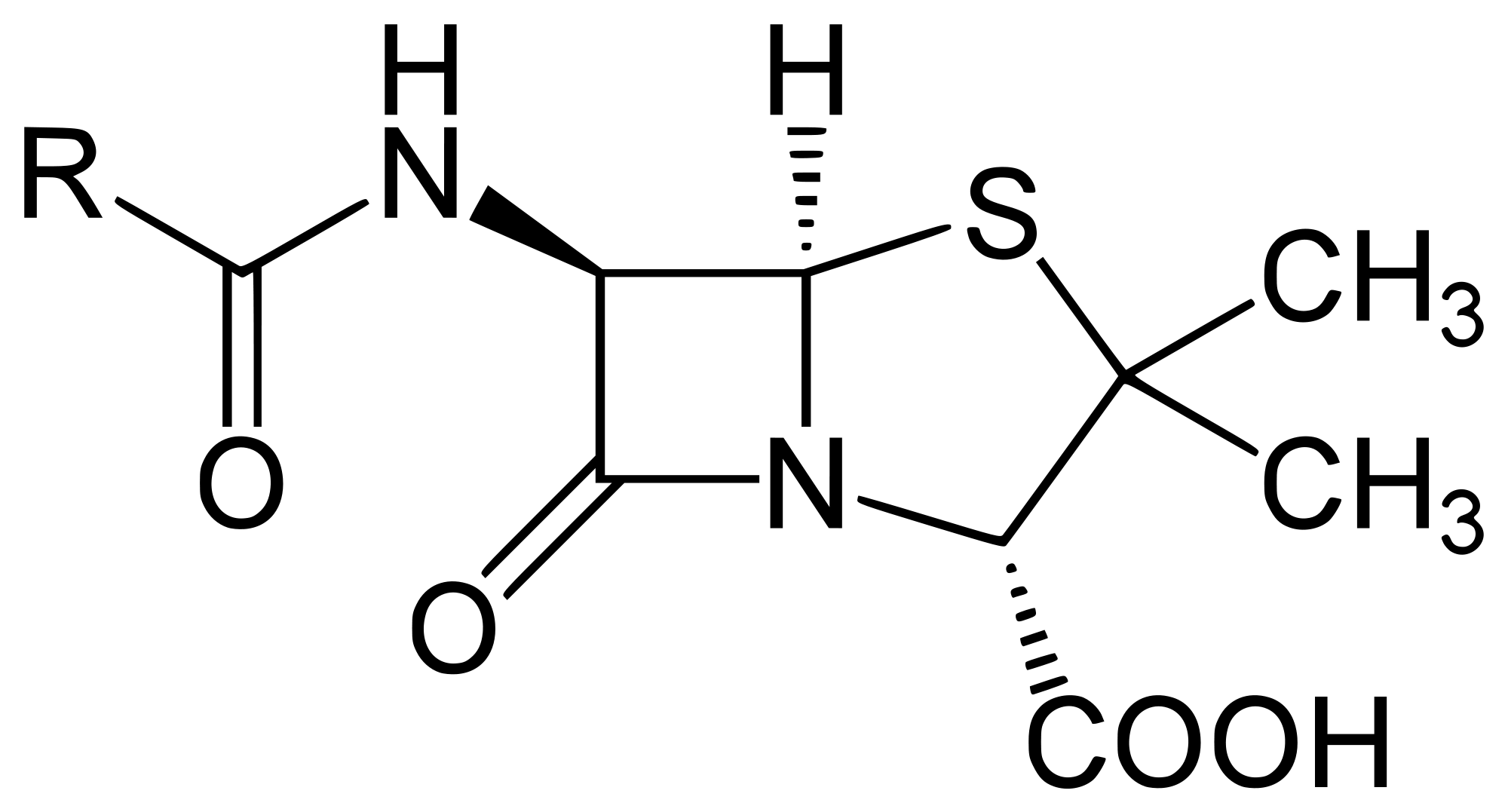
- The term “penicillins” is used as a collective name for a group of structurally similar natural and synthetic substances
Mechanism of penicillin
- A distinctive feature of penicillins is the four membered beta-lactam ring, responsible for antibacterial properties of these drugs
- This beta-lactam ring is highly reactive and irreversibly binds to the enzyme transpeptidase in bacteria. This weakens their cell wall causing water to flow into the bacteria until the water pressure bursts the bacteria open
- Human and other animal cells don’t have cell walls therefore are not affected by penicillin
- Certain bacteria mutated and developed varying degrees of antibiotic resistance due to increased production of enzyme called penicillinase. This enzyme could deactivate penicillin and preventing it from binding to transpeptidase. Over time, this species of bacteria became the dominant species, reducing effectiveness of bacteria
- To overcome this resistance, new penicillins with modified side chains were developed
- This continued production of penicillin triggered multidrug resistance (MDR) in bacteria. MDR bacterial infections require a combination of many different antibiotics and strict patient compliance to solve the issue




