Home 16.1 Rate expression and reaction mechanism
16.1 Rate expression and reaction mechanism
Rate equation (Rate law)
- Consider reaction:

- Where x, y, p, q are stoichiometry coefficients
- The rate equation is:
- In general the rate law is expressed as such:

- Where m and n are the orders with respect to their reactants
- Order of reaction = m + n
- DO NOT USE STOICHIOMETRY COEFFICIENTS AS “m” AND “n” IN THIS EQUATION
- Rate equations can only be determined experimentally because orders can only be deduced empirically
Molecularity and rate-determining step (slow-step) of a reaction
- Reaction mechanism: The sequence of steps outlining the reaction pathway from reactants to products
- Elementary steps: Any individual step in the reaction mechanism
- Molecularity: the number of molecules or atoms involved as reactants in the elementary reaction
- Unimolecular: Single molecule or atoms involved in an elementary step
- Bimolecular: Two molecules or atoms involved in an elementary step
- Each elementary step has its own rate constant, k, and its own activation energy Ea
- The rate of reaction depends on the slow step or the rate-determining step (RDS)
- Catalysts alter reaction mechanism by introducing a step with a lower activation energy
Deduction of a rate equation
- Decide on which step is the RDS
- Deduce rate equation for the RDS
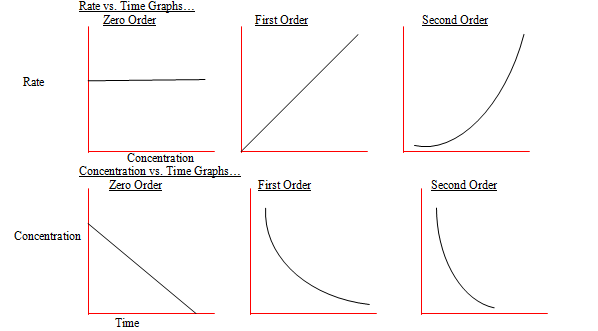
Graphical representations of reactions