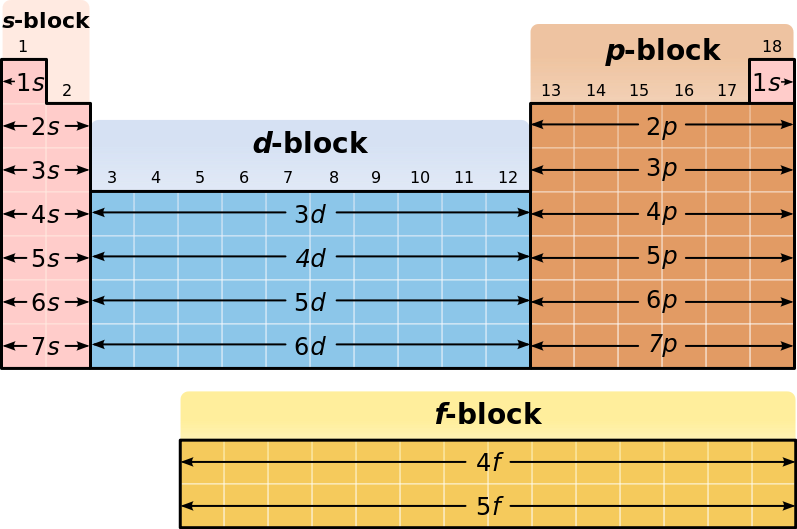
- Groups: Go vertically down
- Periods: horizontal rows of elements
- There are 7 periods. The period number is equal to principle quantum number n of highest occupied energy level
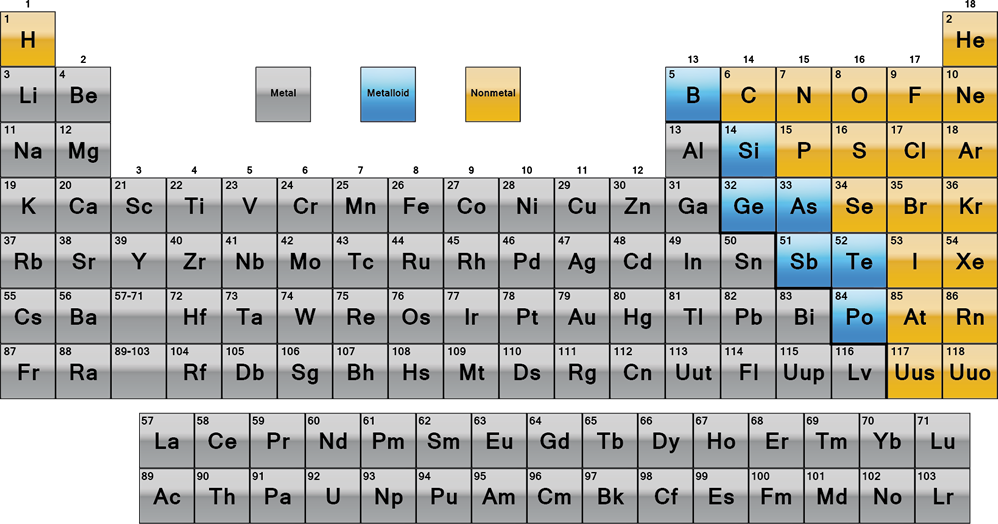
- Periodic table is split roughly into metals, non-metals and metalloids
- Left of line is metals, right of line is nonmetals, touching line is metalloids.
- Metals:
- Good conductors
- Malleable
- Ductile
- Have lustre
- Lose electrons
- Non-metals:
- Poor conductors
- Gain electrons
- Metalloids
- Close to the line, have both metallic and non-metallic properties
- Some metalloids are semi-conductors
- Metals:
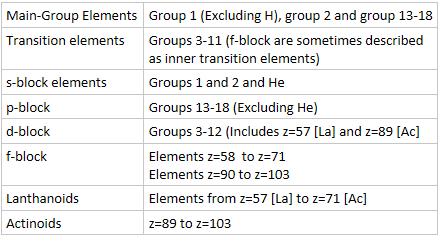
- The periodic table is split into four blocks based on s, p, d, f
- Occupancy of electrons for each sub-level is:
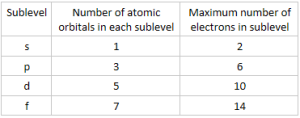
*Two electrons per each orbital
- Number of Valence electron (outer-shell electrons) can be found from the group number of the s and p block elements.