Home 21.1 Spectroscopic identification of organic compounds
21.1 Spectroscopic identification of organic compounds
High-Resolution 1H NMR spectroscopy
- Usually, 1H NMR contain multiple peaks
- In a high resolution H NMR spectrum, single peaks present in low resolution can split into further clusters of peaks
- These multiple peaks result from spin-spin coupling
Main features of 1H NMR spectra
- Number of different absorptions (peaks)
- Corresponds to the number of different chemical environments occupied by protons
- Area under each peak
- Proportional to the number of hydrogen atoms in that chemical environment
- Chemical shift
- Since spinning electrons create their own magnetic field. This results in a lower frequency at which they resonate. This chemical shift is measured in ppm relative to the standard tetramethylsilane (TMS)
- Splitting pattern
- If the number of adjacent protons is equivalent to n then the peak will be split into (n+1) peaks
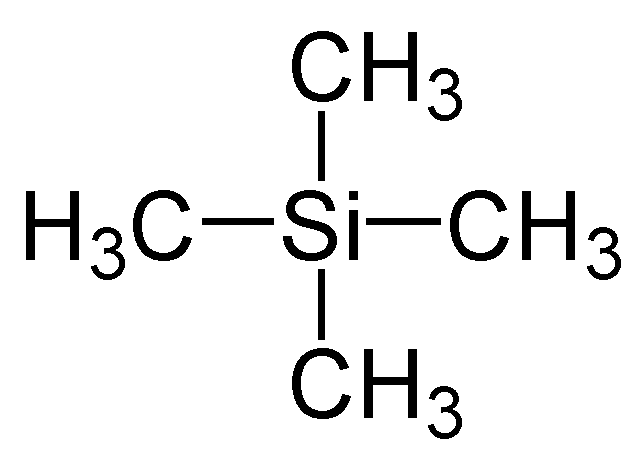
TMS as the standard reference
- All the protons are in the same environment so it gives strong single peak
- It is not toxic and unreactive
- It is volatile; can be easily removed from sample
Single crystal X-ray crystallography
- Single crystal X-ray crystallography can be used to identify the bond lengths and bond angles of crystalline compounds

