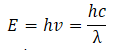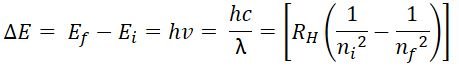Electromagnetic Spectrum (EMS)
- Visible light, radio waves, infrared waves (IR), ultraviolet (UV), x-rays and gamma rays are forms of electromagnetic radiation
- c=vλ where λ is wavelength and v is frequency and c is the speed of light (3.00 x 108)
Emission Spectra:
- When element in gaseous state is subjected to high voltage under reduced pressure, the gas will emit a certain light. When passed through a prism, the spectrum is not continuous but a black background with certain line spectrums
Quantization of Energy
- The line spectrums in the line emission have specific wave lengths λ. Each wavelength coresspons to discrete amount of energy. Quantization is based on this idea that ER comes in discrete packets or “quanta”
- A photon is a quantum of radiation and its energy can be found by equation:
- Energy of electron in a particular orbit is fixed/quantized. The energy of the electron in a particular orbit of a hydrogen atom is given by expression:
- Where:
- RH = Rydberg constant = 2.18 x 10-18 J
- n= principal quantum number, with integers 1,2,3,4… depending on the orbit the electron occupies
- When electron is in ground state is excited, moves to higher excited state, falls back to ground state and emits a photon which is discrete amount of energy. This photon corresponds to particular λ
- The difference in energy between the levels can be
- Hydrogen emission spectrum consists of a series of lines in visible region of spectrum. This is called the balmer series.
- Important transitions
- The line spectrums get closer together (converge) at higher energy levels
Quantum mechanical model of atom
- Schrödinger’s Equation integrates wave and particle nature of electrons
- Solution to equation is mathematical functions called wave functions ψ
- ψ2 represents probability of finding electron in region of space and is called probability density
- These multiple probability density solutions are termed atomic orbitals
- Atomic orbital is region in space where there is high probability of finding an electron.
- There are several types of atomic orbitals, each has a unique shape and associated energy.
S atomic orbital
- Spherically symmetrical
P atomic orbital
- Dumbbell shaped
- Three orientations of p atomic orbitals. There are px, py, pz pointing in directions along Cartesian axes
Energy Levels, sublevels, orbitals , and electron spin
- As n increases, position of electron is further from nucleus and the energies of orbitals increase
- Each energy level can hold maximum number of electrons given by 2n2
- Electron capacity for n=1 is 2, n=2 is 8, n = 3 is 18. This is why we have two elements in first row of periodic table, 8 in second, etc.
- There are energy levels classified by principle quantum number, n, 1,2,3,4,…
- Sub levels are classified by azimuthal quantum number, l, s, p, d, f
- Orbitals which are the actual probability density with electrons. Classified by magnetic quantum number, ml. px, py, pz are examples of 3 orbitals
- Orientation of electron is classified by spin magnetic quantum number ms. There is +1/2, -1/2. Both are arbitrary numbers with no meaning.
Things to know:
-
Aufbau Principle: electrons fill lowest-energy orbital first
-
Pauli exclusion principle: each orbital can hold maximum of 2 electrons, each with opposite spin
-
Hunds rule: When filling degenerate orbitals (orbitals of equal energy), electron fill all orbitals singly before occupying them in pairs
-
Full electron configuration
-
Condensed electron configuration
-
Arrow in box orbital diagram