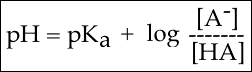Home D.4 pH regulation of the stomach
D.4 pH regulation of the stomach
Stomach Acids
- The process of digestion occurs in the stomach by the fluid known as gastric juice which is composed of water salt (KCl and NaCl), HCl, and enzymes (pepsins)
- The concentration of hydrochloric acid in the stomach varies from 0.003 to 0.1 mol dm-3 which corresponds to a pH of 1.0 to 2.5
- The acid itself does not break down food molecules but it denatures proteins and provide optimum pH for pepsins and other enzymes
- The HCl acts as a disinfectant killing all harmful microorganisms digested with food
Antacids
- Excessive production of hydrochloric acid in the stomach is commonly associated with indigestion
- This causes heartburn nausea and unpleasant feelings which can be reduces by pharmaceutical drugs known as antacids
- Antacids: Quickly increase pH of gastric juice by reacting with hydrochloric acid
- Common antacids are hydroxides, carbonates, hydrogencarbonates of calcium, magnesium, and aluminum
- As with any pharmaceutical, they have side effects
- Aluminum hydroxide reduces the concentration of phosphates in body fluids, while carbonates and hydrogen carbonates produce carbon dioxide. Intake of calcium, magnesium and sodium ions affect the electrolyte balance in the body and can lead to various conditions, ranging from diarrhea and constipation to heart failure
- Antacids are often combined with anti-foaming agents which relive bloating by allowing bubbles of CO2 to coalesce and leave body
Regulation of acid secretion
- The acidity of gastric juice can be controlled at cellular level by targeting biochemical mechanisms of acid production
- The secretion of acid in stomach is triggered by histamine binding to H2-histamine receptors in cells of gastric lining
- Ranitidine (Zantac) blocks H2-histamine receptors and reduce secretion of acid. It also provides short term relief of symptoms of indigestion and require frequent administration
- Omeprazole (Prilosec) and esomeprazole (Nexium) reduce production of stomach acid by inhibiting enzyme known as gastric proton pump, which is directly responsible for secreting H+ ions into gastric juice. In contrast to ranitidine, these provide longer relief of up of three days
- Omeprazole and esomeprazole have same molecular formula (C17H19N3O3S) and differ only in their stereoisomeric structure
- These structures are chiral and exist as two enantiomers
- Omeprazole is a racemic mixture of both enantiomers while esomeprazole is a single enantiomer
- Both enantiomers have low polarity and thus easily cross cell membrane, and undergo chemical transformations in the acidic environment to produce active metabolites which then bind to proton pump enzymes. This increases efficiency of drug and thus requires less frequency of administration
Acid-base buffers
- Acid-base buffers prevent a change in pH when a small amount of acid or base is added to solution
- Buffers consist of strong base and excess weak acid or strong acid and excess weak base
- Weak acid and bases exist in equilibrium, thus have a K constant
- This can be rearranged to form the Henderson Hasselbach equation
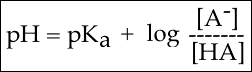
- Where A– is conjugate base and HA is the acid
Hydrogencarbonate and carbonate buffers
- Primary buffer system in body is carbon dioxide and hydrogencarbonate ions
- Hydrogen carbonates have multiple protons and this have a pKa1 and pKa2 value
Buffer pH range
- At pH = pKa a buffer solution reaches maximum efficiency and can neutralized the most
- Its most effective in the range of pH = pKa -1 to pH = pKa + 1