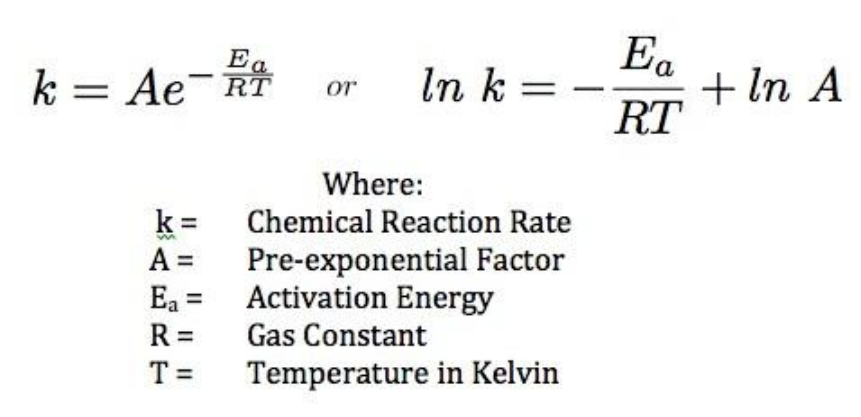Arrhenius Equation
- Uses temperature dependence of the rate constant to determine activation energy
- The “Frequency factor”, A, takes into account the frequency of collisions with proper orientations
- The graph of lnk against 1/T is a linear plot with gradient -Ea/R and intercept lnA
- Only way to learn this is to do practice questions