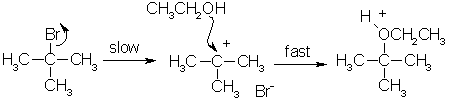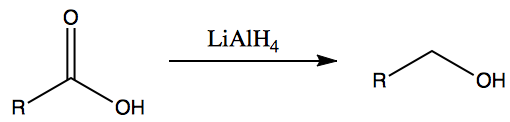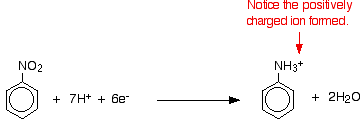Home 20.1 Types of organic reactions
20.1 Types of organic reactions
Overview
- Nucleophilic Substitution
- Sn2
- Sn1
- Factors affecting rate of nucleophilic Substitution
- Electrophilic Addition
- Hydrogen halides
- Halogens
- Interhalogens
- Electrophilic Substitution
- Nitration of Benzene
- Reduction
- Conversion of nitrobenzene to phenylamine
Nucleophilic Substitution Reactions
- The chloroalkane produced after free radical substitution mechanism has very different properties from those of the alkane. This is due to highly electronegative chlorine bonded to C creating a polar bond
- The carbon is thus susceptible to attack by nucleophiles which are electron rich species capable of donating a pair of electrons
- Good nucleophiles
- Ones which can donate electron pair easily
- H2O vs OH– . OH– is a stronger nucleophiles because it is negative and thus greater attraction for electron deficient species (electrophiles)
- There are 2 types of nucleophilic substitution (explained in detail below)
- SN2 which is for primary halogenoalkane
- SN1 which is for tertiary halogenoalkanes
- The reason for the two types
- Inductive effects: the bonds to alkyl groups in the carbon atom have different inductive effects. Tertiary compounds are more stable and thus go through SN1 mechanism while primary compounds are less stable and go through SN2 mechanism
SN2 reactions and primary halogenoalkanes
- Bimolecular meaning 2 reactants (molecule and nucleophile)
- This is a concerted reaction meaning it is a 1 step reaction where reactants are converted directly to products
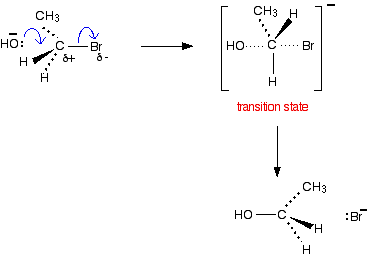
- Hydroxide is nucleophile with electron pair
- Attacks the partially positive carbon atom from the backside forming a transition state in which both are attached to the carbon atom. In transition state, nucleophile and leaving group are 180° to each other.
- When nucleophile enters the molecule, a Walden inversion in which the shape of the molecule inverts. Similar to analogy of inverting umbrella
- The products are thus stereoisomeric
- Rate Determining Step: depends on concentration of the halogenoalkane and nucleophile
- Rate = k[halogenoalkane][nucleophile]
SN1 reactions and tertiary halogenoalkanes
- Unimolecular (one reactant)
- Has 2 steps
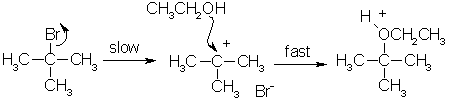
- Rate Determining Step: depends only on concentration of the halogenoalkane
Factors affecting rate of nucleophilic substitution
- Identity of halogen
- Presence of good leaving group results in increased rate of reaction in both mechanisms
- e.g. CH3I reacts faster than CH3F
- Class of halogenoalkane (Primary, secondary, tertiary)
- Class has direct effect in rate of formation of carbocation (rate-determining step)
- Tertiary compounds have SN1 mechanism. Also greater stability due to inductive effects. They are faster because less activation energy
- Secondary compounds can have both SN1 /SN2 mechanism
- Primary compounds have SN2 mechanisms. They are slower.
- Choice of solvent
- SN2 best performed in aprotic, non-polar solvents. (No OH or NH groups thus no hydrogen bonding, no polar). Example is propanone.
- SN1 best performed in protic, polar solvents. (Hydrogen bonding, polar). Example is water.
Electrophilic addition reactions
- Electrophile is an electron deficient species that will accept a pair of electrons. Example is nitronium ion NO2+
- Electrophiles are Lewis Acids
- Electrophiles can either be cations with positive charge, or have partial positive charge (δ+)
- Doubles bonds are more susceptible to electrophilic attack
- Electrophiles are used in addition reactions to form alkenes
- Markovnikov rule: the electrophile bonds to the carbon in the double bond with the least number of hydrogens bonded to it.
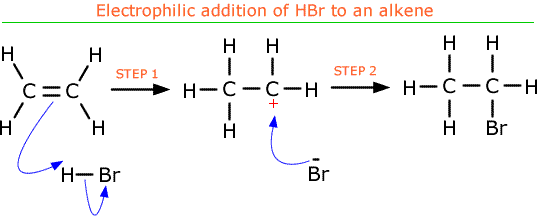
- Electrophilic addition of hydrogen halides
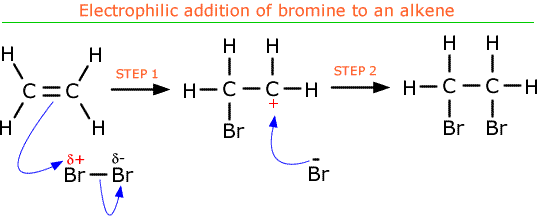
- Electrophilic addition of halogens to alkenes
- Electrophilic addition of interhalogens to alkenes
- Same as the above accept you have to determine which halogen is partially positive and partially negative
- The partially negative atom is what bonds to the cation
- Br—Cl
- δ+ —δ–
Electrophilic substitution reactions
- Benzene does not undergo addition, that’s why there is electrophilic substitution
- Each carbon to carbon bond of benzene has a bond order of 1.5
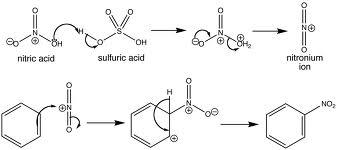
- For IB, have to know nitration of benzene. There are steps in this reaction.
- Generate the NO2+ ion
- Reaction with benzene ring
Reduction
- This is the reverse of mild oxidation reactions of alcohols
- Carboxylic acids can be reduced to aldehydes which can then be reduced to primary alcohols.
- Ketones can be reduced to secondary alcohols.
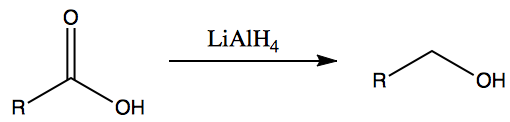
- LiAlH4 can be replaced by [H] to represent reduction agent
- Have to know the conversion of nitrobenzene to phenylamine. This is a two stage process.
- Reduction of Nitrobenzene into phenylammonium ions
- Nitrobenzene is reduced to phenylammonium ions using a mixture of tin and concentrated HCl
- Mixture is heated under reflux
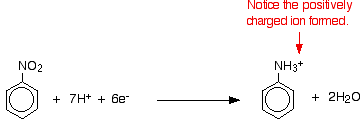
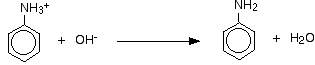
- Formation of phenylammonium to phenylaniline
- Involves removing H+ from -NH3 group
- Done in sodium hydroxide solution (NaOH)