Periodic Trends
Electron configuration help determine atomic properties. Atomic properties show pattern amongst the period table which can be classified as trends
6 trends need to know:
- Atomic Radius
- Ionic Radius
- Ionization Energy
- Electron Affinity
- Electronegativity
- Metallic vs non metallic
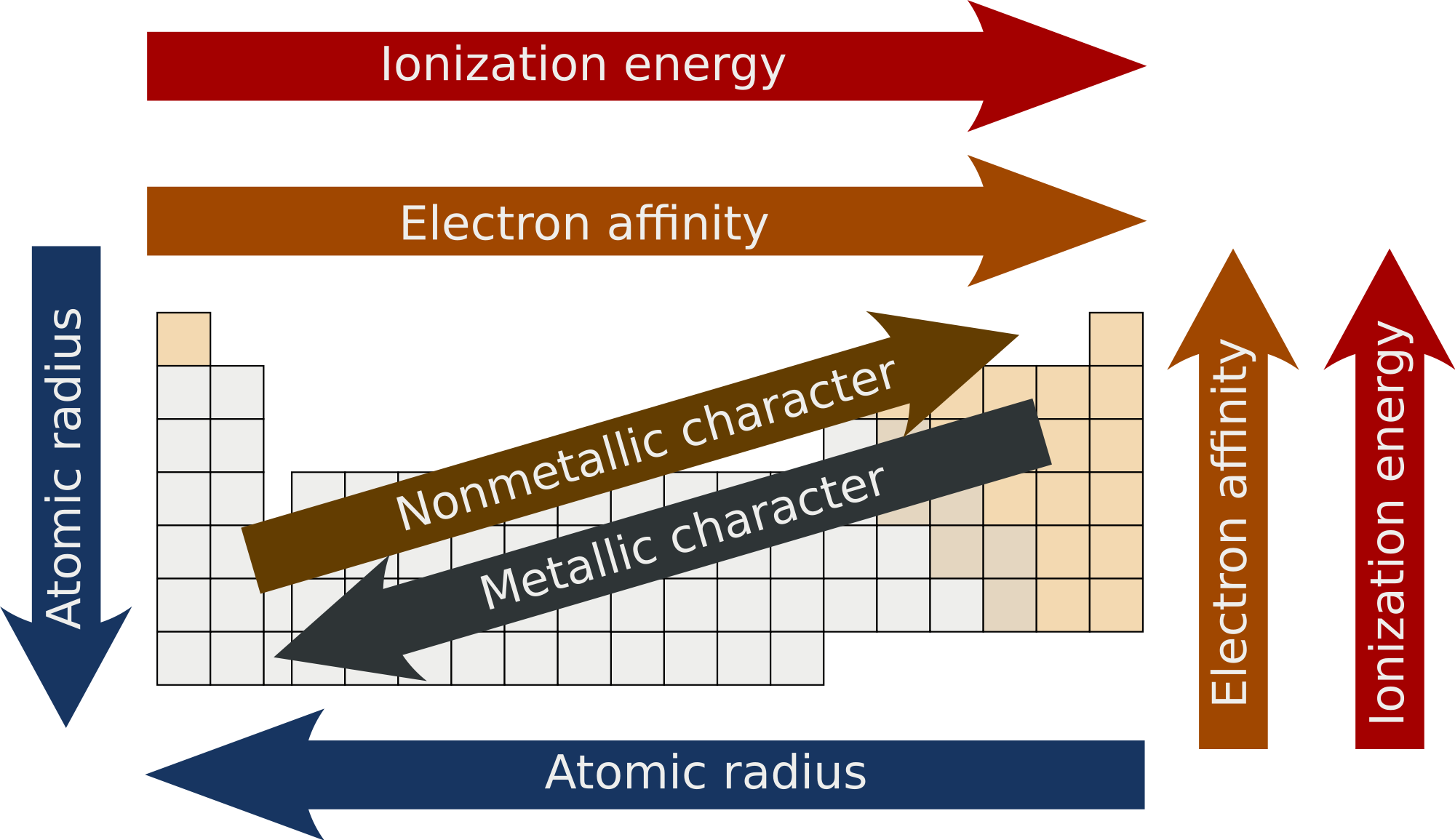
- Atomic Radius
- Definition
- Distance from center to the outer most region
- Bohr model has orbits, thus radius can be measured easily however we know that this is highly simplistic. Electrons are found in orbitals
- Way of finding radius would be to measure distance between two atoms in diatomic molecule X2 and divide by two for the radius. R = 1/2d
- Trends
- Atomic radius decreases across period from left to right. Nucleus’s positive charge is greater relative to charge of electrons in same shell, pulling electrons closer to nucleus and thus reducing atomic radius
- Atomic radius increase down group from top to bottom. Each new period begins with new energy level which is farther from nucleus, increasing atomic radius.
- Definition
- Ionic Radius
- Definition
- Distance from center to the outer most region in ion
- Radii of cations and anions are different from parent atoms
- Trends
- Radii of cations (e.g. Mg+) are smaller than their parent. This is because there are more protons than electrons in the cation, so valence are more strongly attracted to nucleus.
- Radii of anions(e.g. Cl–) are larger than their parent. This is because the extra electron in the anion results in greater repulsion between valence electrons, thus larger radius.
- Definition
- Ionization Energy (IE)
- Definition
- Second ionization energy IE2 is energy required to remove electron from IE1
- Positive sign meaning energy used (endothermic)
- Trends
- IE increases across period from left to right. This is because since electrons are pulled closer to nucleus (see atomic radius), more difficult to remove them.
- IE decreases down a group from top to bottom. This is because atomic radii increase down group, making easier to remove electron. Also because of shielding effect weakens forces between nucleus and outer electrons.
- Electron Affinity
- Definition
- The change in energy released which accompanies addition of an electron to an atom in gaseous state.
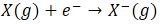
- Negative sign meaning energy released (exothermic)
- Trends
- Increases across a period from left to right (becomes more negative. With some exceptions)
- Decreases down a group from top to bottom (becomes less negative. With some exceptions)
- Definition
- Electronegativity
- Definition
- Relative attractions that an atom has for a shared pair of electrons in a covalent bond
- Trends
- Same as ionization energy for same reasons
- Electronegativity increases across period from left to right. This is because since electrons are pulled closer to nucleus (see atomic radius), more difficult to remove them.
- Electronegativity decreases down a group from top to bottom. This is because atomic radii increase down group, making easier to remove electron. Also because of shielding effect weakens forces between nucleus and outer electrons.
- Definition
- Metallic & Non-metallic
- Definition
- These trends occur due to combination of trends above
- Trends
- Metallic character decreases across period from left to right
- Metallic character increases down a group from top to bottom
- Tend to have low IE values – tend to lose electrons. Thus they are oxidized
- Definition
Trends in properties of metals and non-metal oxides
- Oxide is formed from combination of element with oxygen
- Metal Oxides + water –> base
- Non-metal oxide + water –> acid
- SiO2 classified as acidic oxide, because it can react with NaOH to form Na2SiO3 (aq) and water.
- Al2O3 is classified as amphoteric oxide. It can react as both an acid and a base.
Similarities and differences in properties of elements
- Group 1: More vigorous reactions with water as you go down the group
- Group 17: Less vigorous reactions going down the group. Reason being atomic radius increases, harder to gain electron

