Home 13.2 Coloured complexes
13.2 Coloured complexes
- In isolated atom, d orbital has 2 energy levels. In complex ion they split into two sublevels. Electronic transitions between these sublevels leads to absorption and emission of photons of visible light.
Crystal Field Theory (CFT)
- D sub-level consists of five d orbitals dxy, dyz, dxz, dx2-y2, dz2
- In free metal ion, Mn+, with ligands infinite distance away, these five orbitals are degenerate (same level)
- If electrostatic field created by the ligand point charge is spherically symmetrical, energies of d-orbital will remain degenerate but increase in energy
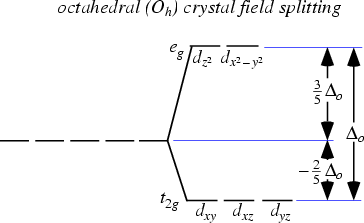
- If electrostatic field created by ligand is octahedral, d orbitals will split into two sets of degenerate energy, the t2g set and the eg T2g set will decrease in energy (stabilize). Eg will increase in energy (destabilized)
- t2g includes dxy, dyz, dxz,
- eg includes dx2-y2, dz2 which point at axis ligands are on
- Crystal Field, field caused by negatively charged ligands, which cause loss of degeneracy
- After 3 spots are filled in t2g, it can choose to go to higher energy level eg, where destabilized. Or take more energy to fill half full t2g This energy is called P, pairing energy.

- Factors affecting crystal field splitting energy
- Identity of metal ion
- Δo increases, when descending a group with metal in same oxidation state
- Oxidation state of metal ion
- Δo increases as oxidation state increases
- Nature of ligands
- Ammonia has greater charge density than water
- Geometry of complex ion
- Δt for tetrahedral complex is about 4/9 Δo
Explanation of colour transition metal complexes
- Colour comes from partially filled d orbital
- Transition metal complexes absorb complementary colours to what they transmit
- When electron jumps from lower to higher energy, requires photon of energy and thus emits light


