Skip to content
Home 19.1 Electrochemical cells
19.1 Electrochemical cells
Voltaic Cells
- Electromotive Force (EMF): The energy supplied by a source divided by the electric charge transported through the source
- In voltaic cell, a cell potential is generated, resulting in movement of electrons from anode to cathode via external circuit.
- Cell potential: the potential difference between the cathode and the anode when the cell is operating
- Under standard conditions, cell potential is called Standard Cell potential
- In order to calculate Eᶱcell for a spontaneous cell, the cathode is taken as the more positive value from the two electrodes
- The more positive one is also the strongest oxidizing agent
Standard Hydrogen Electrode (SHE)
- Consists of an inert platinum electrode in contact with 1 mol dm-3 hydrogen ions and hydrogen gas at 100 kPa and 298 K. This is an example of a gas electrode
- Standard electrode potential of a single half-cell cannot be measured on its own. Has to be relative to another cell
- Standard electrode potentials are measured relative to SHE
- SHE has Eᶱcell of 0V
- The reduction half equation corresponding to the SHE cell is
Cell potential and Gibbs free energy
- Spontaneous:
- Eᶱcell is positive, ΔG is negative
- Non-Spontaneous
- Eᶱcell is negative, ΔG is positive
- When ΔG is 0, Eᶱcell is 0
- Both are related by following equation:
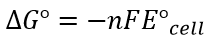
- Where:
- n= amount, in mol, of electrons
- F = Faraday’s constant = 96500 C mol-1
Electrolytic Cells
- Convert electrical to chemical energy
- In SL, we looked at electrolysis of molten salt, now we will look at types of electrolysis
- The higher the reduction potential, higher the tendency to react
- Electrolysis aqueous NaCl
- Concentrated
- You have to take into account water as well
- At cathode, water is reduced to create hydrogen gas
- At anode, Cl– is oxidized to create Cl2 gas
- Diluted
- At cathode, hydrogen ions are reduced to create hydrogen gas
- At anode, water is oxidized to produce oxygen gas
- This is equivalent to electrolysis of water
- Electrolysis of CuSO4
- Inert graphite (carbon) electrodes
- Electrodes don’t take part in reactions
- At cathode, copper ions are reduced to create copper deposits
- At anode, water is oxidized to produce oxygen gas
- Active copper electrodes
- Electrodes take part in reaction
- At cathode, copper ions are reduced to create copper deposits
- At anode, sludge of impurities is found
- Process known as electrorefining in which the impurities in copper are separated from copper itself
- Also the basis of electroplating in which a thin layer of metal is deposited onto cathode of another
- Electrolysis of water
- Water is poor conductor of electricity
- Electrolysis of water is done in dilute solutions of sulfuric acid or sodium hydroxide using inert Pt electrodes
- At cathode, hydrogen ions are reduced to create hydrogen gas
- At anode, water is oxidized to produce oxygen gas
Factors affecting amount of product formed
- Current
- Higher the current, greater yield
- Q=It
- Duration of electrolysis
- Longer the time, greater yield
- Charge on the ion
- Na+ required 1 mol of electrons however Pb2+ requires 2 mols of electrons