Skip to content
Home 4.3 Covalent structures
4.3 Covalent structures
Lewis (Electron dot) structures
- Based on formation of covalent bonds in a molecule
- Important to show:
- Bonding pairs of electrons (showing covalent bond as single, double, or triple bonds
- Non-bonding pairs of electrons (often called lone pairs, not involved in bonding)
- Each line represents 1 pair of electrons (2 electrons)
- Each dot represents 1 electron
- The Octet Rule states that elements tend to lose electrons, or gain electrons, or share electrons in order to acquire noble gas configuration (8 electrons in valence shell)
Lewis (Electron dot) structures of cations, anions and ionic compounds
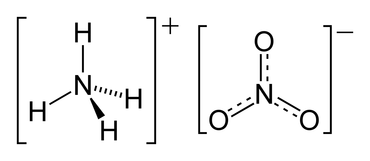
- When you have a bond between cation [NH4]+ and anion [NO3]–, you have ionic bond between the ions, however the cation and anion are covalent in nature
- Here is diagram of ammonium nitrate NH4NO3
Valence Shell Electron Pair Repulsion [VSEPR] theory
- Lewis structures don’t tell anything about actual shape. They are 2D
- VSEPR theory takes into account 3D shape and bond angles
- Theory is: since electrons are negatively charged subatomic particles, pairs of electrons repel one another to be as far apart as possible in space
- Every pair of electrons are described as occupying an electron domain
- Electron Domain Geometry based on total number of electron domains
- Molecular Geometry gives shape of molecule
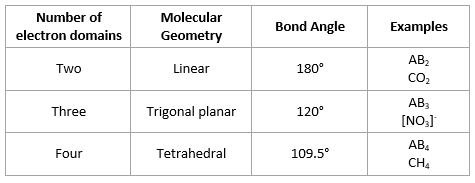
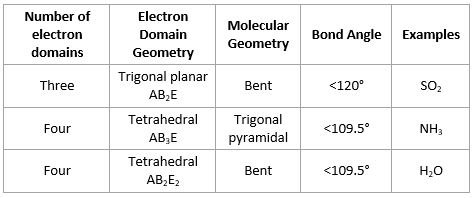
Bond angles in molecular geometries
- Lone pairs occupy more space than bonding pairs, so they decrease bond angle between bonding pairs
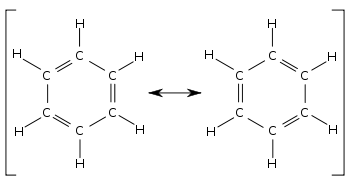
Resonance Structures
- Occurs when there is more than one possible arrangement for a double bond in a molecule
- These multiple possible arrangements contribute to an overall structure called resonance forms.
- The actual electronic structure of the species is called a resonance hybrid of these resonance forms.
- In order to show this idea of resonance, the contributing resonance forms are linked via a double arrow
- One best known example of resonance is benzene C6H6 which forms delocalized benzene ring
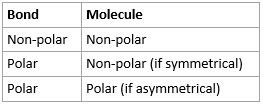
Molecular Polarity
- Whether a molecule itself is polar or not
- Easy method:
- If there are non-polar bonds, molecule is non polar
- If there are polar bonds and molecule is symmetrical, molecule is non-polar
- If there are polar bonds and molecule is asymmetrical, molecule is polar
Allotropes
- Allotropes of same element can vary in both chemical and physical properties
- Carbon has 4 allotropes: graphite, diamond, graphene, C60 fullerene
- Covalent Network Solids is one with atoms held together by covalent bonds in 3D lattice structures.
- Covalent Network Solid examples include graphite, diamond, graphene, and SiO2
- C60 is molecular because had weak London forces between molecules
Silicon Dioxide
- Silicon dioxide in its most common crystalline form is called quartz. This is another example of a three-dimensional covalent network solid
Coordinate Covalent Bonding
- The shared pair of electrons comes from only 1 of the 2 atoms
- Examples include [NH4]+





