Three types of intermolecular forces of attraction
- London forces (also called dispersion forces or instantaneous induced dipole-induced dipole forces)
- Dipole-dipole forces
- Hydrogen bonding
Van der waals forces = London forces + dipole-dipole forces + dipole-induced dipole
- London Forces
- H2 for example. Although molecule is non-polar, every H2 molecule consists of positive nuclei with cloud of negatively charged electrons. If you were to take a snapshot at any given instant of time, one part of molecule may have slightly more electron density than another part. This generates a temporary dipole. It is termed an instantaneous dipole
- What affects magnitude of London forces?
- Number of electrons
- Greater the number of electrons, the larger the distance between the valence electrons and the nucleus. Attraction of valence electrons to nucleus will be reduced and hence the electron cloud will be polarized more easily increasing the boiling point.
- Size (volume) of the electron cloud
- In a large electron cloud, the attraction of electrons to the nucleus will not be as great as in smaller cloud, hence the electrons in larger clouds can be polarized easily increasing boiling point.
- Shapes of molecules
- Larger the surface area of molecule, the more area of interaction with other molecules increasing the boiling point.
- Number of electrons
- Dipole-dipole Forces
- Exists in all polar molecules permanently
- When positive end of one dipole is attracted to negative end of one dipole
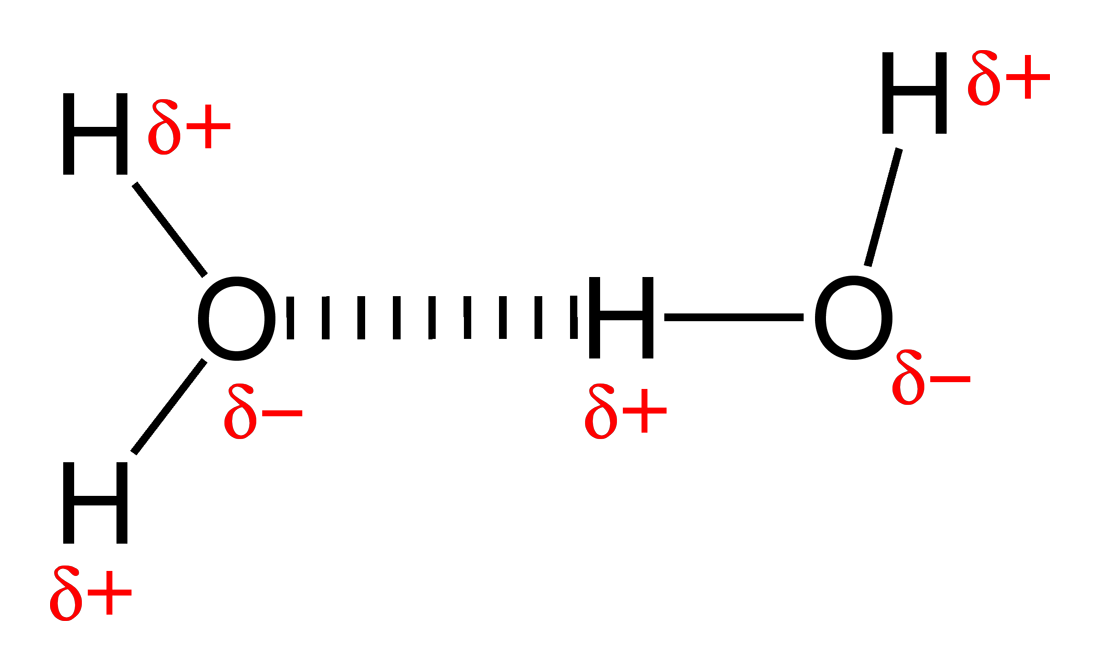
- Hydrogen Bonding
- Occurs only between hydrogen and F, O or N
- Occur between molecules
- Examples include hydrogen bonding in water
London Forces < dipole-dipole forces < hydrogen bonds