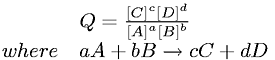Equilibrium Reactions in Chemistry
- Many chemical reactions are reversible and exist in state of equilibrium
- Dynamic Equilibrium: The forward and reverse reactions occur at equal rates
- Single arrows are for non-reversible reactions
- Double headed arrow are for reversible reactions which are in equilibrium
Chemical Systems
- The terms “Reactants” and “Products” implies a reaction goes to completion when in reality, many reactions are in equilibrium
- Relative rates of a reaction depend on:
- Temperature and Pressure
- Concentration of reactants and products
- Presence of catalyst
- At equilibrium:
- Forward and reverse reactions occur at equal rates
- No change in concentration of reactants or products
Equilibrium Law
- Law: At a given temperature, the ratio of the concentration of products to the concentration of reactants (each raised to the power of their molar coefficients) is a constant
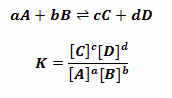
- Constant is called equilibrium constant denoted by Kc
- This constant changes at different temperatures
- Large Kc value (Kc > 1) means products are favored over reactants
- Small Kc value (Kc < 1) means reactants are favored over products
- Pure solids and liquids are not included in when calculating the Kc value
- Homogenous equilibrium: reactants and products are present in one phase. (Common one is reactions in gaseous phase)
- Heterogeneous equilibrium: reactants and products exist in more than one phase
- Kc of the reverse reaction is the same as 1/Kc of the forward reaction
- Kc (reverse) = 1/Kc (forward)
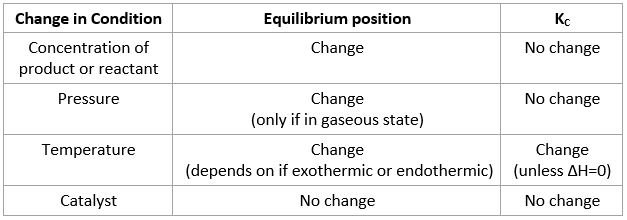
Effect of conditions on equilibrium constant
Pressure:
- If there are 4 mols of gaseous reactants and 3 mols of gaseous products, an increase in pressure would result in forward reaction being favored to reduce number of mols overall
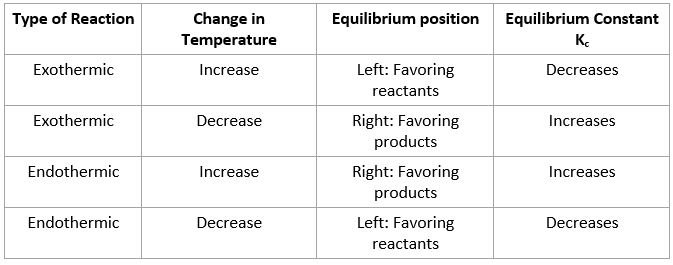
Temperature:
- In exothermic reaction, heat is a product. Increase in heat would cause equilibrium position to move left to counter the imbalance caused by heat. A summary of all possibilities is in table below
Catalyst:
- Catalyst reduces activation energy
- It increases rate of forward and reverse reaction by equal amount thus no change in Kc
Le Châtelier principle
- Le Châtelier principle: If a change is made to a system that is in equilibrium, the balance between the forward and reverse reactions will shift to offset this change and return system to equilibrium
- If concentration of reactant is increased, forward reaction will be favored to counteract this. Vice Versa
- Value of Kc does not change
Reaction Quotient
- If system has not reached equilibrium yet, then
- Q is for reaction quotient and helps determine the progress of reaction as it moves toward equilibrium