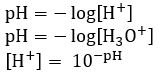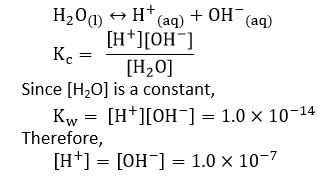The pH scale
- pH scale is effective way of representing concentration of hydrogen ions [H+] in a solution
- It is a logarithmic scale with base 10, easy way for non-scientist to understand safety of materials
- pH distinguishes between acidic, neutral and alkaline
Calculating pH
- The concentration of an acid with one proton is the same as the concentration of hydrogen ions [H+]
- [HCl] = [H+]
- For more protons:
- [H2SO4] = 2[H+]
Ionization of water