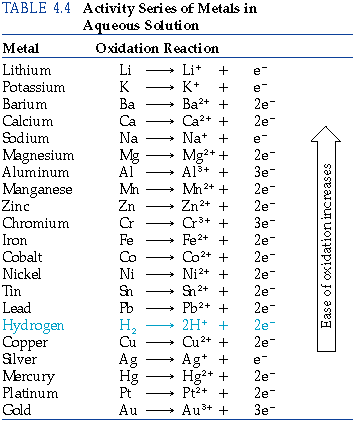Home 9.1 Oxidation and reduction
9.1 Oxidation and reduction
Redox Reactions
- Redox reactions are a type of chemical reactions which involves reduction and oxidations
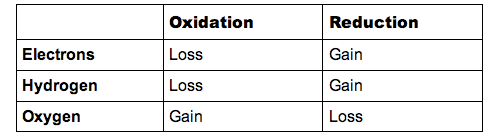
- A useful mnemonic for remembering how redox processes work is:
- OIL RIG
- Oxidation Is Loss (of electrons) ; Reduction Is Gain (of electrons)
Oxidation States
- The Oxidation state is the apparent charge of an atom in a free element, molecule or ion.
- In terms of oxidation state:
- Oxidation describes a process in which the oxidation state increases
- Reduction describes a process in which the oxidation state decreases
- To determine if a reaction is a redox reaction, observe if there is a change in oxidation state for each element.
- Elements in their natural states and carbon dioxide are considered to have oxidation number of 0
- Rules for oxidation states:
- Charge always goes before the number
- 0 for an atom in a free element (Na, O2, S8)
- +1 for group 1 metals. +2 for group 2 elements
- +1 for hydrogen when bonded to a non-metal. -1 when bonded to a metal in hydride form (NaH)
- -2 for oxygen except in any peroxide where it is -1
- -1 for group 17 halogens. Oxidation state is positive in combination with oxygen in oxoanions and oxoacids (Cl has oxidation state of +7 in HClO4)
- In polyatomic ions, sum of oxidation states of all atoms equals overall charge of the ion
Variable Oxidation States
- In addition to the rules for oxidation states, there are elements with variable oxidation states. These consist mainly of transition elements
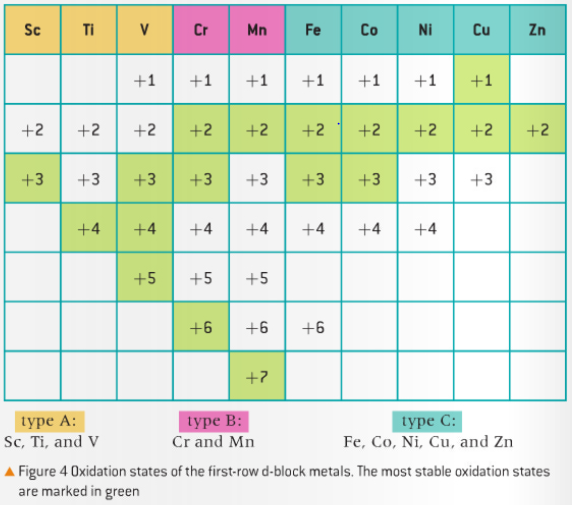
- Since compounds with transition metals have variable oxidation states, the roman numeral system is used to name such compounds according to IUPAC
- Oxidation numbers are used for transition metals while oxidation states are used for all other elements
- g. KMnO4 is potassium manganate (VII) as Mn has an oxidation number of +7
Balancing Redox Reactions
- Half equations are used to balance complex redox reactions
- Only required to balance an equation in acidic or neutral media
- Steps to balance equations:
- Assign oxidation states for each atom in the reactant and product species
- Deduce which species is oxidized and which species is reduced
- State the half-equation for the oxidation process and the corresponding half-equation for the reduction process
- Balance these half-equations so that the number of electrons lost equals the number of electrons gained
- Add the two half equations together to write the overall redox reaction
- Check the total charge on the reactant and product sides
- Balance charges by adding H+ and H2O to the appropriate sides
The activity series
- The activity series ranks metals according to the ease with which they undergo oxidation
- Most reactive metals are found at the top of the series
- Series found in section 25 of the data booklet
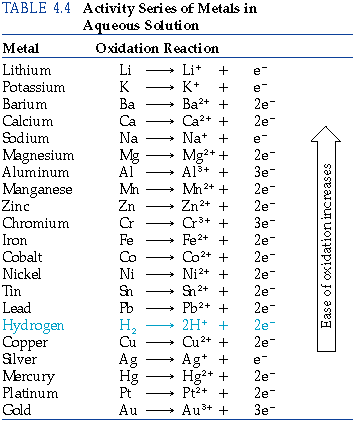
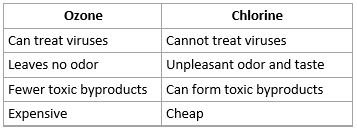
Use of chlorine and ozone as disinfectants in drinking water
- Access to clean water is determined to be a human right, yet over a billion people do not have this
- Water supplies are normally disinfected using strong oxidizing agents such as chlorine (Cl2) or ozone (O3) to kill microbial pathogens
- Chlorine can be added in three forms: Cl2, NaOCl and Ca(OCl)2. All three solutions yield HOCl which is the antibacterial agent
- The use of chlorine can also cause problems for people. Some people object the taste of water. Chlorine can also react with other chemical to from toxins such as chloroform CHCl3
Redox Titrations
Winkler Method and BOD
- The amount of oxygen dissolved is used to indicate the quality of a body of water
- The Winkler Method is a technique used to measure the amount of oxygen dissolved in water
- In general, a high concentration of dissolved oxygen indicates a low level of pollution, thus higher quality of water
- Biochemical oxygen demand (BOD) is the degree of organic pollution in a sample of water
- BOD is defined as the amount of oxygen required to oxidize organic matter in a sample of water at a definite temperature over a period of 5 days. BOD is measured in units of ppm.
- Pure water generally has BOD less than 1 ppm