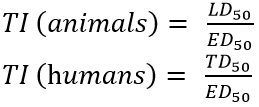Home D.1 Pharmaceutical products and drug action
D.1 Pharmaceutical products and drug action
Introduction to medicinal chemistry
- Medicinal chemistry is a link between organic chemistry, pharmacology, biochemistry, biology and medicine
- Primary objective is to produce compounds called pharmaceutical drugs which have a variety of effects on the human body including curing diseases, or assisting in medical diagnostics
- Therapeutic effect: the desirable outcome of a pharmaceutical drug on the body
- Placebo: biologically inert substance which achieves therapeutic effect due to the body’s natural healing process
- Placebo effect: the actual therapeutic effect achieved by a placebo
- Side effect: Non-desirable outcomes associated with a pharmaceutical drug
Routes of Administration
- Orally
- Rectally (in the form of suppositories or enemas)
- Parentally (under the skin, subcutaneous injection)
- Into muscles tissue (intramuscular injection)
- Into bloodstream (intravenous injection, produces fastest therapeutic effect)
- Inhalation (breathed in through nose or mouth)
- Transdermally (applied to skin in forms of patches or ointments
Effectiveness and Safety
- The effectiveness and safety of a drug can be expressed using its therapeutic index (TI)
- Effective dose (ED50): the minimum dose of the drug required to produce the desired therapeutic effect in 50% of the subjects
- Lethal dose (LD50): the minimum dose of the drug that causes death in 50% of the subjects
- Toxic dose (TD50): the minimum dose of the drug required to cause toxicity in 50% of the subjects (TD50 used because unethical to kill humans to determine LD50)
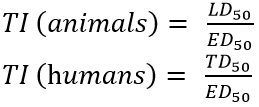
- The higher the TI, the safer the drug
- Therapeutic Window: the range of doses where the drug provides the desired therapeutic effect without causing unacceptable adverse effects in most patients
- In contrast to TI, therapeutic window is not strictly defined and only serves as a general indication
- In general, window opens before ED50 and closes before TD50
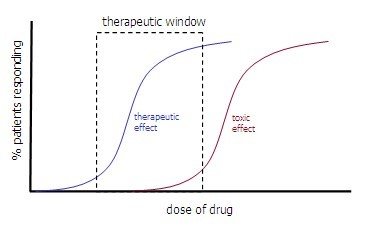
- Effective and toxic dose of drug depend on its route of administration. In order to reach target organ, has to go through bloodstream, which is problematic if drug insoluble
- Drug bioavailability: the fraction of administered dose that reaches target part of body
- By definition, intravenous injection has 100% drug bioavailability while other methods of administration decrease in bioavailability
- Bioavailability of pharmaceutical drugs depend on their solubility, polarity, and the presence of certain functional groups. More soluble with polar groups.
Tolerance and addiction
- Drug Tolerance: reduce the body’s response to specific medications due to accelerated drug metabolism. This is typical for opiates and other narcotic drugs where users need progressively higher doses to obtain desired effects. Increased dosage leads to more side effects
- Drug Addiction: The compulsive desire of the user to take drugs regardless of the health problems it might cause
- Drug addiction becomes dangerous when combined with drug tolerance
Drug action and development of new drugs
- At the molecular level, pharmaceutical drugs interact with binding sites of enzymes or cellular In binding to enzymes, most drugs act as inhibitors
- The type and efficiency of drug-receptor interactions depend on chemical structure of drug and the site of activity
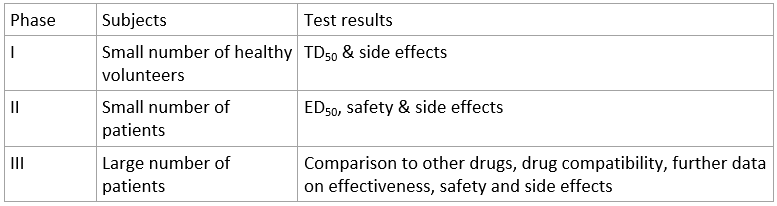
- Drug Development is long and complex process which takes many years
- Drug Discovery:
- First step is identification of a lead compound (or new chemical entity, NCE) which shows promising activity towards a specific biological target. The lead compound is then isolated and screened against bacteria or animals
- Very slow, expensive and inefficient process
- Drug Design:
- Relies on knowledge about drug-receptor interactions. If chemical composition and three dimensional structure of a particular biological target are known, a molecule with complementary structure can be designed using computer modelling techniques. The designed molecule is then synthesized and tested. If any difference occurs, the computer model can easily be adjusted and tried again.
- Preclinical trials: After lead compound is identified, each compound is rated according to toxicity, chemical stability, solubility, preparation costs, and many other properties that may be desirable. The drug must also cause no side effects to other molecules and have minimal environmental impact in production
- Clinical trials: Set of steps after the success of preclinical trials which includes testing on humans
- Post-clinical trials (Phase IV): For determining the long term effects and chronic toxicity of the drug, including carcinogenic properties, effect on immune system, fertility, and reproductive functions